Organizations using both a RIM Submissions Vault and a Clinical Operations eTMF Vault on the same domain can use a standard Vault to Vault connection. The RIM-Clinical Operations connection transfers Product Family, Clinical Study, and Site records across Vaults and automates the creation, versioning, and updating of CrossLink documents and document fields. Streamlining object record and document creation reduces duplicate data and allows Vault documents to have a single source of truth within an organization.
Note: The RIM-Clinical Operations Connection is only available for organizations with both a Submissions Vault and eTMF Vault on the same domain.
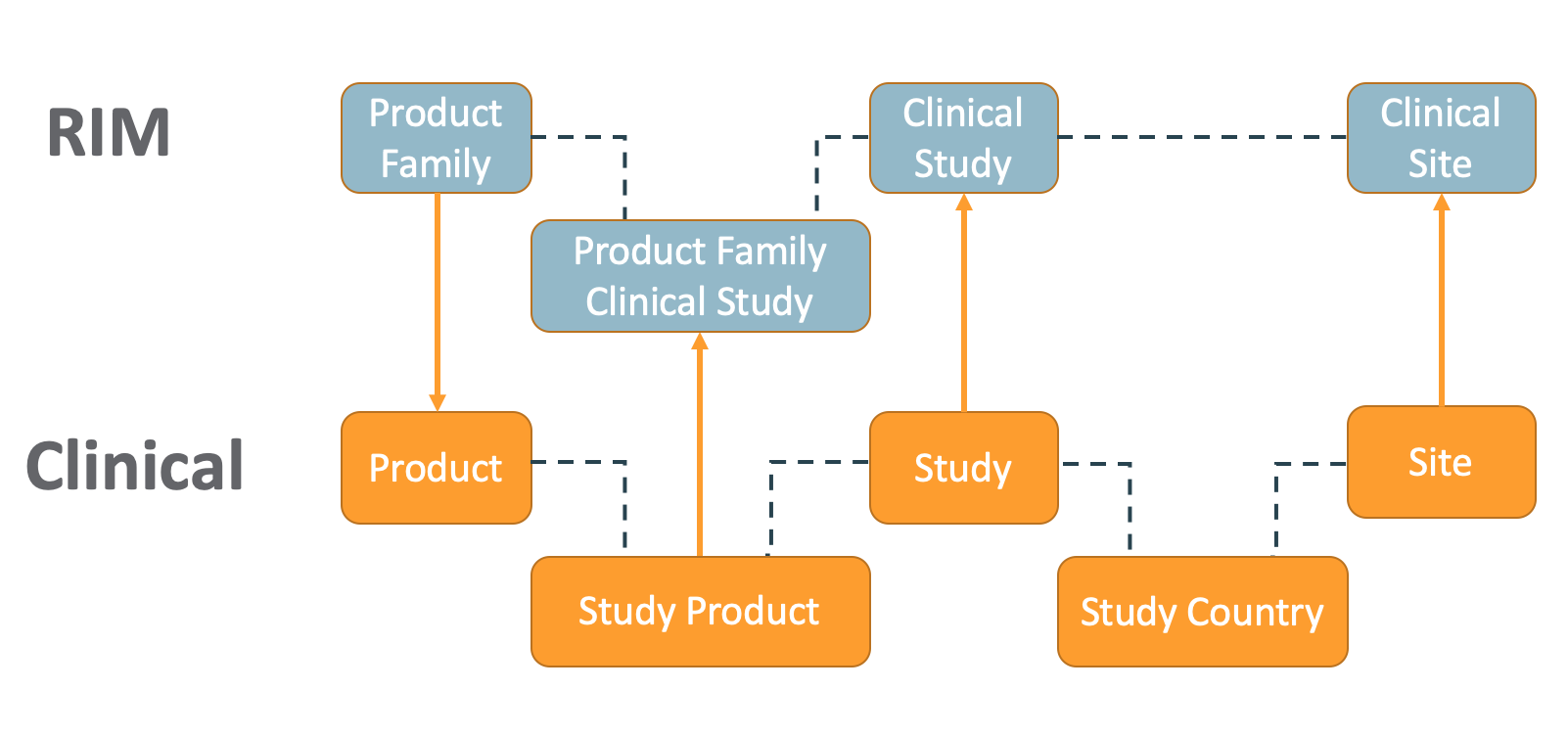
How the RIM-Clinical Operations Connection Works
The RIM-Clinical Operations Connection creates new records, updates existing records, and creates CrossLink documents across Vaults in the following situations:
- When a user creates or edits a Product Family record in the Submissions Vault, Vault creates or updates the Product record in the eTMF Vault.
- When a user creates or edits a Study record in the eTMF Vault, Vault creates or updates the Clinical Study record in the RIM Vault.
- If the Study Type, Study Subtype, or Type of Control fields are populated on the Study record, Vault updates the Clinical Study record with the appropriate mapped fields in RIM.
- When a user creates or edits a Study Product record in the eTMF Vault, Vault creates or updates the Product Family Clinical Study record in the RIM.
- When a user creates or edits a Site record in the eTMF Vault, Vault creates a Clinical Site record under the same Clinical Study record in RIM and populates the Site Country field based on Study Country in the eTMF Vault.
- If the RIM source document to be crosslinked has a value of European Union in the Country field, then Vault maps all relevant Study Countries where the Country belongs to the Jurisdiction European Union to the Clinical Crosslink. To utilize this mapping logic, configuration of the Jurisdiction Country object is required. Reach out to Support for the recommended mappings.
- When a document enters its Steady state in the source Vault, Vault creates or versions a CrossLink document in the target Vault and populates fields. Admins can also configure Vault to transfer document versions to the connected Vault when they reach the source Vault’s Superseded state. An Admin defines CrossLink document behavior in the connection’s configuration. See details about document creation below.
- If there is no Study field populated on the RIM document to be CrossLinked, Vault automatically populates the Study field in the Clinical Operations Vault based on the Active Lead Agent Product associated with the RIM document.
- The Connection treats documents as having no Study populated if the study’s Link field in RIM is not populated with a valid study from Clinical Operations, or if the Study field is left empty on the document.
- If there is a populated Study field on the RIM document to be CrossLinked, Vault automatically populates the Study field in the Clinical Operations Vault with the value on the RIM document as long as the study is valid in Clinical Operations.
- If the Redacted field is populated on the Clinical document to be CrossLinked, Vault automatically populates the field in the RIM Vault with the value on the Clinical document.
Connection behavior considerations:
- Once the connection creates a CrossLink and populated the Study field as described in the last two above scenarios, if a new Study Product record is created in Vault eTMF, then the connection will not automatically reprocess any CrossLinks to add additional Studies to documents. Users must modify the source documents in RIM in order for metadata sync to occur.
- When users delete documents or object records in one Vault, Vault does not delete the associated documents or object records in the connected Vault.
About Record Creation & Update
Vault creates new records in their lifecycles’ Starting states. When creating a record through the connection, Vault also populates the Link (link__sys) field on the target record with the source record’s Global ID (global_id__sys). These fields let Vault know which records to update in the target Vault when data is updated in the source Vault.
About Document Creation & Update
Vault creates new CrossLink documents in the steady state defined in the target Vault’s document lifecycle of a given document type. If the document’s type has multiple lifecycles, Vault applies the first lifecycle available. The connection creates CrossLink documents using the Specific Document Version binding rule. See details about source binding rules below.
The RIM-Clinical Operations Connection creates, up-versions and syncs metadata for documents in the target Vault that are configured to transfer between Vault applications:
- When a source document enters its Steady state, the Connection creates a CrossLink in the target Vault in the Steady state of the target’s Document Type defined on the Document Lifecycle. Vault also applies any entry actions configured on that lifecycle state, for example, setting the version number to a major version.
- If a Connection-created CrossLink exists on the target Vault and a new version of the source document is created and moved to a steady state, the Connection up-versions the Target CrossLink and creates a new CrossLink version.
- When the fields on a Steady state source document are modified in the source Vault, Vault updates those fields on the CrossLink document in the target Vault.
- If the connection has never run, Vault only transfers the latest Steady state version of the document.
- Document versions created in the target Vault are set by the target Vault and bound to the versions from which they were created in the source Vault.
- When enabled by an Admin, Vault transfers any Superseded versions of a document created since the last time the connection ran.
- If there are both Steady state and Superseded versions of the source document in the source Vault that have not been transferred to the target Vault since the last time the connection ran, Vault transfers all of these document versions.
- The Documents Integration is triggered when (1) a source document in-scope for transfer enters the Steady State and (2) a source document that has a Connection-created CrossLink has a metadata update. Updates on an Approved source document that does not have a Connection-created CrossLink does not trigger the Connection.
Note: Vault does not apply document reuse functionality to CrossLink documents in eTMF Vaults that were transferred from Submissions Vaults.
About Source Binding Rules
If a CrossLink document does not already exist in the target Vault, Vault creates the CrossLink document and binds it to the current document version in the source Vault. Vault will then update the CrossLink document only if a user in the source Vault specifically edits the version of the source document that is bound.
All CrossLink documents created via the RIM-Clinical Operations Connection have the Source Binding Rule set to Specific Document Version.