The Study Person object automates the creation of User Role Setup records according to rules specific to your Vault. Study Person records are leveraged across the Clinical Operations product suite and are used to manage document reuse across studies and access to archived studies.
Admins can set up a system where Study or Country Managers easily maintain their own team rosters, supporting a quicker study start up process and easier upkeep. Additionally, Responsibilities can be assigned to Study Persons directly from Study Person object records.
Configuring Dynamic Access Control for Documents on Study Personnel
The Study Person object allows you to utilize the automation capabilities of your Clinical Operations Vault and the flexibility of Dynamic Access Control (DAC) for documents to avoid manually creating hundreds or thousands of User Role Setup records.
Admins can configure Study Team Roles that map to application roles and role dependencies to Study Team Role records. Additionally, users can restrict the assignment of Study Team Roles based on Person Type and/or Security Profile. When you attempt to save a record, Vault performs validation checks to prevent users from creating Study Person records with disallowed combinations.
Note: For security reasons, the maximum number of Study Team Roles allowed in a Vault is 300. If you attempt to create an additional Study Team Role and there are already 300 records in your Vault, Vault issues an error message and does not allow you to continue.
Example
Tracy is a Site CRA for Site 101. For this role, she needs Editor access to all Site 101 documents, but also needs Viewer access to country-level and study-level documents. Without Study Person, an Admin would manually set up three (3) User Role Setup records for Tracy. With this feature configured, the Study Manager could create a single Study Person record with the Grant Access to Related Records (create_urs__v) field set to Yes indicating that Tracy is a Site CRA responsible for Site 101. Based on the configuration, Vault would automatically create all three User Role Setup records.
When Tracy moves to a new position midway through the study, the process for removing her access is also simple. The Study Manager simply updates the Grant Access to Related Records field to No and then deletes or inactivates the Study Person record.
As an additional layer of security, Vault does not allow any changes to the Study Person, Study Team Role, Study, Study Country, and Site records while the Grant Access to Related Records (create_urs__v) field is set to Yes.
How to Create Study Persons
How to create a Study Person record:
- Navigate to the Study Personnel tab.
- Click Create.
- Select a Person.
- Select a Study Team Role.
- Select a Study and then select a Study Country and Study Site, if needed.
- Set the Grant Access to Related Records field to Yes.
- Select the Survey Respondent Type from the picklist. For surveys of the type you select here, Vault uses the Study Person’s email address when sending the survey if the Study Person is associated with the survey.
- Click Save.
If you select Principal Investigator for the Study Team Role field and select a Study Site, Vault automatically adds that person to the Principal Investigator field in the Details section of the Study Site record. If the Study Site record already has a Principal Investigator and your administrator has enabled Sync PI Field with Study Person, the new person replaces the existing person in the Principal Investigator field. If Sync PI Field with Study Person is not enabled, Vault only updates the Principal Investigator field if it is blank. This update does not occur if the site is in an Archived lifecycle state.
Vault automatically creates the User Role Setup records. Depending on the activity in your Vault, it may take a while before the user has access.
If Sync PI Field with Study Person is enabled and you add a Person to a Study Site’s Principal Investigator field, Vault creates a new Study Person record with the Principal Investigator role for that Person if a record does not exist. Vault also populates the Primary Contact Information field on the new Study Person record with the available Contact Information record when only one (1) record exists for that Person and the Pre-default on non-required field when only one reference record is available setting is enabled on the Study Person object’s Primary Contact Information field.
Note: If the Study Person object contains required custom fields, Vault will still create the Study Person record but will not populate the fields.
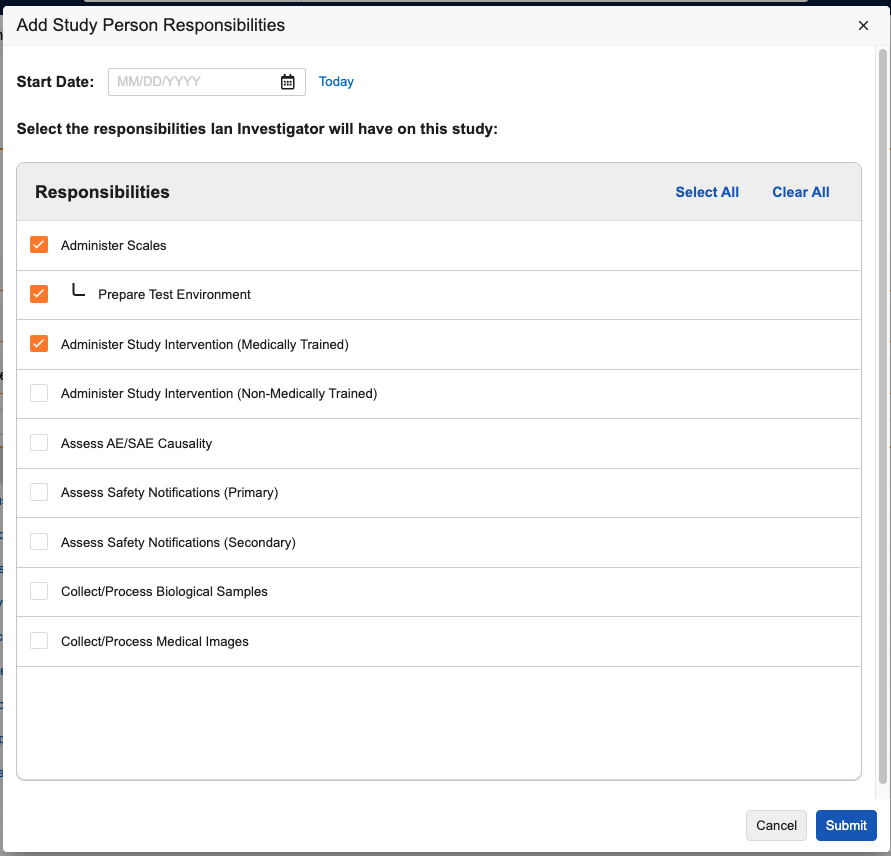
Adding Study Person Responsibilities
Vault supports assigning one (1) or more Responsibilities to Site Staff or Investigator type Study Personnel, along with the duration the person is performing that task. These are defined as Study Person Responsibility object records and can be added to Study Person records using the Study Person Responsibilities application section.
You must assign Study Personnel to a Study Site before you can add Responsibilities. A Responsibility must have a Study Responsibility record for the associated Study before it can be assigned to a Study Person.
To add a Responsibility to a Study Person:
- Navigate to the Study Person record.
- Click Add in the Study Person Responsibilities section.
- In the dialog, select the Responsibilities you want to add. To add a Specialized Responsibility, you must select the parent Standard Responsibility.
- Optional: Assign a Start Date. This will apply to all selected Responsibilities.
- Click Submit.
Note: Users can only have either the Assess Safety Notifications (Primary) or Assess Safety Notifications (Secondary) responsibility, but not both.
Once you click Submit, Vault creates a Study Person Responsibility record for each Responsibility selected. To review and manage Study Person Responsibility records for a Study Person in bulk, click the Show in Tab button in the Study Person Responsibilities section of that Study Person record.
Enter an End Date on the Study Person Responsibility record to remove the Responsibility from the Study Person. Once the End Date for the Study Person Responsibility record is in the past, the Responsibility can be reassigned to the Study Person. If you select the Responsibility for a Study Person who has the Responsibility assigned in an existing Study Person Responsibility record with an End Date, Vault clears the End Date for the Study Person Responsibility record.
Customers with connected Study Training Vaults can transfer Study Person Responsibility records to Study Training to define Responsibility-based training.
Applying End Dates to Study Person Responsibility Records
When you enter an End Date for a Study Person with Study Person Responsibility records, the Apply End Date to Study Person Responsibility Records job applies the End Date to the Study Person Responsibility records if the Person does not have any other Study Person records with no End Date for that Study Site. If the Person has another Study Person record with no End Date for the Study Site, the job does not apply an End Date to the Study Person Responsibility records. The Apply End Date to Study Person Responsibility Records job runs daily.
The Apply End Date to Study Person Responsibility Records job is inactive by default. We recommend Admins enable the job to benefit from the streamlined Responsibility experience.
Removing Access for Study Personnel
The Revoke Access from Study Persons with End Date job removes a user’s access to study-related records when their Study Person record reaches the End Date. When active, the Revoke Access from Study Persons with End Date job runs daily and automatically changes the Grant Access to Related Records field to No for Study Person records where the End Date is before the date the job runs. This allows the Study Person record to remain active while removing access to related records.
For Vaults with Site Connect, the Revoke Access from Study Persons with End Date job also removes the user’s access to Site Connect by changing the Site Connect User field to No.
Admin can navigate to Admin > Operations > Job Definitions to activate the Revoke Access from Study Persons with End Date job.